Geographic atrophy (GA) is a progressive retinal disorder leading to irreversible vision loss.1 GA lesion progression is slow, with lesions typically enlarging and coalescing to include the fovea within 2.5 years.2,3 Growth rate is variable, meaning patients need close monitoring; patients may have stable vision with not much lesion growth for months or years, only to then experience rapid vision loss.4 Although a cure for GA remains elusive, we now have two FDA-approved treatments that slow GA progression: pegcetacoplan and avacincaptad pegol (ACP).5,6 To be effective, it is critical that clinicians intervene at the appropriate time. The following educational activity provides a comprehensive discussion on diagnosing GA, referring patients, and when to initiate treatment.
DIAGNOSING AND IMAGING GEOGRAPHIC ATROPHY
Using OCT to Diagnose GA
GA lesions can be identified through several testing and imaging modalities such as optical coherence tomography (OCT), dilated fundoscopic exam, and fundus autofluorescence (FAF).1 OCT has near-infrared or blue-infrared imaging that can be used to find areas of atrophy. Specific OCT biomarkers that suggest progression to GA are:
- Reticular pseudodrusen (RPD)/subretinal drusenoid deposits (SDD)
- Hyperreflective foci and columns
- Hyperreflective and hypertransmission columns7,8
Drusen and pigment epithelial detachment (PED) resemble a bumpy line (Figure 1A). When these suddenly drop out, like Figure 1B, GA is possible.

Figure 1. Example of OCT biomarkers suggesting progression to GA. Images courtesy of Mohammad Rafieetary, OD.
Figure 2 shows reticular pseudodrusen and subretinal drusenoid deposits. It’s important to know if it’s above or below the retinal pigment epithelium (RPE). In Figure 2, the reticular pseudodrusen are above the level of the RPE.
Figure 3A shows incomplete RPE and outer retinal atrophy (iRORA), which appear as little spots of incomplete atrophy. They’re less than 250 μm and have dropout areas. You can see hypertransmission into the choroidal layer on Figure 3B. Clinicians sometimes call the multiple vertical lines, which indicate the signal is getting all the way through, a barcode sign.
Complete RPE and outer retina atrophy (cRORA) has a few diagnostic criteria9:
- Zone of hypertransmission of at least 250 μm
- Zone of RPE attenuation or disruption of at least 250 μm
- Evidence of overlying photoreceptor degeneration
- No signs of scrolled RPE or RPE tear
An important takeaway for diagnosing cRORA is that if the patient has an RPE tear, then it’s not GA. The RPE tear resembles GA—where you can see right through it—but is caused by the missing RPE.

Figure 2. Example of reticular pseudodrusen and subretinal drusenoid deposits. Images courtesy of Mohammad Rafieetary, OD.

Figure 3. Example of iRORA. Images courtesy of Mohammad Rafieetary, OD.
Using FAF to Diagnose GA
I obtain a high-quality FAF on every advanced AMD patient. I don’t obtain it on every visit, but if I notice signs of GA on the OCT and suspect it’s getting worse, I recommend exploring it further on the FAF. True atrophy shows up as an area of hypoautofluorescence or darkness. Around that, look for bright hyperautofluorescence because autofluorescence can be from lipofuscin redirecting your light source back up. When you see that brightness, it’s indicating that the cells are stressed and more likely to die off soon.
Figure 4 is a good example of this. It shows three spots of missing cells seen as hypoautofluorescence and a few speckled spots surrounding the missing cells. Another important aspectof atrophy is the placement. Is it in the center of the fovea or are there multiple spots?
Biomarkers and predictors of atrophy on FAF are:
- Size (small vs large)
- Configuration (unifocal vs multifocal)
- Location (with or without subfoveal involvement)
- Fluorescence pattern (none, focal, patchy, banded, diffuse, trickling)
- Is the lesion right next to the macula or is it in a pattern that looks like it will come into the center?

Figure 4. Example of hypoautofluorescence on FAF. Images courtesy of Mohammad Rafieetary, OD.
Figure 5 shows an example of hypoautofluorescence and surrounding bright hyperautofluorescence.
The hyperautofluorescent edges on Figure 5 will likely become more of the dark area within the next year as these stressed cells become fully atrophic. Our available medications are thought to slow that progression and protect those cells by inhibiting the complement cascade.
Figure 6 demonstrates the difference between color fundus photography (CFP) and FAF. On CFP, the atrophy is not always clear. After examining Figure 6A, you expect to see a big spot temporally (evident in Figure 6D) in Figures 6B and 6C. This can be difficult to see as the GA is only partially visible in Figure 6B and 6C. However, the FAF images show that there is more dropout than was evident from the three CFPs. I obtain an FAF before I treat any patient with GA medications. If I’m unsure about how the disease is progressing, I’ll obtain an FAF and repeat it 3 or 6 months later, depending on my level of concern.
It’s important to note that old scars, an old RPE tear, or a spot of blood may look like dropout/atrophy but without the surrounding hyperautofluorescence. You must always correlate it back to your exam. I’ve also seen several patients with GA who have had a spot, without the typical hyperautofluorescence around it, for a long time. In those cases, I do not treat right away or at all. Often, the patient will return 3 or 6 months later, and the spot looks the same.

Figure 5. Example of hypo- and hyperautofluorescence on FAF. Images courtesy of Mohammad Rafieetary, OD.

Figure 6. Lesions on CFP (A-C) vs FAF (D-F). Images courtesy of Mohammad Rafieetary, OD.
UNDERSTANDING GA PROGRESSION
The AREDS study first showed that the average time for the extrafoveal GA to encroach into the fovea was about 2.5 years.3 Approximately 57% of those people will develop central GA within 4 years.10 The median rate of GA progression is 1.78 mm2/ year, although it varies from patient to patient and is dependent on lesion parameters and fellow-eye status.11,12 Visual function drops precipitously when the fovea becomes involved.
A retrospective cohort analysis of a multicenter UK EMR assessed patients who were 50 years of age or older with bilateral GA and no history of choroidal neovascularization (CNV). They found that progressive vision loss leads to a considerable proportion of patients losing their ability to drive.13 A total of 67% of patients became ineligible to drive due to progressive vision loss over a median of 1.6 years. It took a median of 6.2 years for 16% of patients to become legally blind. The lesson here is that if you don’t intervene, GA will progress.
A retrospective observational study from the American Academy of Ophthalmology Intelligent Research in Sight (IRIS) Registry analyzed 593,277 patients who were diagnosed with dry AMD between January 2016 and December 2019 to further understand progression in these patients.14 The researchers characterized dry AMD by distribution of visual acuity categories and evaluated visual acuity progression risk by disease stage. At baseline, 64.4% had mild disease, 29.4% intermediate, and 2.9%/3.3% had GA with/without subfoveal involvement. At the end of the 4-year study, they found that patients with mild AMD progress to intermediate disease about 12% of the time. Patients with GA without subfoveal involvement progress to the GA with subfoveal involvement about 11% of the time.
WHEN IS IT TIME TO REFER?
When do you refer a patient with GA? Is it when central GA lesions have already caused significant loss of visual function? Or do you refer when the patient has extrafoveal lesions that are not yet a threat to central visual acuity? Here are a few cases of clear referrals.
Case 1 is a 77-year-old pseudophakic white male who had a VA of 20/30 OU at presentation. Figure 7 shows his imaging at baseline and 2 years later. There’s clear progression. The baseline imaging has a clearer central zone, whereas the follow-up imaging shows patches coming in at all sides. The follow-up OCT shows RPE and choriocapillaris dropout, the barcode sign, and subretinal drusenoid deposits.


Figure 7. Case 1: Baseline and follow-up imaging with progression for 77-year-old male. Images courtesy of Roger Goldberg, MD.

The next case is a 90-year-old white female. The OCT shows some diffuse hypertransmission centrally, but no classic GA on the exam or near-infrared reflectance imaging. However, the presence of GA is marked on the FAF, and the historic FAF shows marked progression over the prior 3 years (Figure 8).
Between October 2020 and April 2023, you can see that the GA significantly progressed. Ideally, you would have caught that progression between these time periods to slow it down.




Figure 8. Case 2: Historic and follow-up imaging for 90-year-old female. Images courtesy of Roger Goldberg, MD.
Case 3 is an 87-year-old white female with counting fingers VA OD, but 20/30 VA OS. Figure 9 shows her disease progression between 2017 and 2023, when she was referred. The 2017 image show some patches, which are clearly larger in 2020 and 2023.
A referral in 2017 would have resulted in the best outcomes for this patient, but the GA medications were not available at that time. The spots are very close to her central vision. Treatment may be able to slow progression. The OCT from 2023 shows the dropout of the retina.

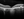
Figure 9. Case 3: Disease progression in 87-year-old who needed earlier referral. Images courtesy of Roger Goldberg, MD.
Case 4 is a 74-year-old white man who works full time. His VA is counting fingers OD from wet AMD and 20/30 OS. He has difficulty driving, especially at night, and feels colors are fading. Figure 10 shows his imaging between 2021 and 2023, illustrating some dropout of the outer plexiform layer as well as the outer nuclear layer. There is clear disease progression between 2021 and 2023.
The take-home points from these cases are that now that we have treatments that slow progression, we need to catch disease early. I recommend referring when there are any subtle signs of atrophy developing on GA (eg, hypertransmission defects, iRORA), even when you can’t visualize the GA on the exam. If you see any signs of dropout or observe a big change in vision, it’s time to refer. Treatments don’t reverse disease. We won’t be able to reverse vision loss, but we can slow progression. There’s not much we can do for a patient who has end-stage GA.



Figure 10. Imaging for a 74-year-old with progression. Images courtesy of Roger Goldberg, MD.
MANAGING PATIENTS WITH GA
Patient selection for treatment with ACP or pegcetacoplan is more complex than ever. Ideally, we would have 6 to 12 months of historical imaging to measure the rate or progression.
This is what I take into account when determining when to treat:
- Size of the lesion
- Distance to the fovea
- Phenotypes (eg, drusen type, multifocality, circularity, FAF pattern, choroidal thickness)
- Photoreceptor loss
- Associated CNV
- Fellow-eye involvement
- Patient comorbidities
GA drugs increase the risk of CNV, and some clinicians may choose to treat GA in the presence of CNV. Because GA treatments are monthly or every other month, patients require frequent appointments. Do your patients have comorbidities that will make that appointment burden challenging?
How GA Impacts Patient Quality of Life
It’s important to appreciate how dry AMD and GA affect patient quality of life. Patients with these diseases tend to get less exercise and have less engagement with friends. They have trouble doing household chores and are, therefore, less likely to invite friends over. It’s too much effort for them to prepare for an outing, so they experience further social isolation. They also need to carry magnifiers, lose reading as a hobby, and are unable to drive.15,16
Patel et al performed a cross-sectional study with a retrospective chart review involving patients 70 years old and older with bilateral symptomatic GA due to AMD.17 Among patients who had a driver’s license, 50% said they did not feel confident driving during the day, and 88% said they did not feel confident driving at night. The majority of patients—82%—reported a worsening of vision during the past year.
Patient and Caregiver Education
When educating patients and their caregivers, it’s important to provide a personalized explanation of AMD that is clear and free of jargon.18,19 Review the causes of AMD and impact on vision in plain language, using visual aids, diagrams, and charts to help patients understand the anatomy of the eye and how AMD affects it. I like to show patients a model of the eye because many people do not understand where the retina is located. Patients also respond well to OCT images.
Explain the different stages of AMD (early, intermediate, and advanced) as well as the two forms of advanced AMD: wet and dry (GA). Keep this high level because it can be too much information for some patients. Also, describe the potential progression of the disease and the associated changes in vision. Focus the discussion on the common symptoms (blurry or distorted vision, difficulty reading, and changes in color perception) and encourage regular self-monitoring through the use of home monitoring devices, such as an Amsler grid or an electronic home monitoring device.
Discuss treatment options and treatment frequency with patients, making sure that they understand that treatment will slow lesion growth but won’t recover lost vision. Be especially mindful when discussing the potential benefits and risks of the treatment because you may give them a reason not to attend the next appointment. Make sure to discuss appropriate timing and urgency for referral to a retina specialists and the importance of adherence to the treatment plan.
Patients with low vision will need to be introduced to low-vision aids (magnifiers, task lighting, and contrast-enhancing devices) and techniques that may enhance their quality of life. Lifestyle modifications, such as improved lighting, high-contrast materials, and large-print books, can help patients adapt to their vision changes.
UNDERSTANDING CLINICAL TRIAL DATA SUPPORTING GA TREATMENTS
There are two FDA-approved complement inhibitor therapies for GA: pegcetacoplan and ACP.5,6 Pegcetacoplan (15 mg intravitreal injection every 25 to 60 days), an intravitreal C3 and C3b inhibitor, was approved in February 2023 for GA secondary to AMD. ACP (2 mg monthly intravitreal injection), an intravitreal C5 inhibitor, was approved for GA secondary to AMD shortly thereafter in August 2023. Pegcetacoplan was approved based off OAKS and DERBY data, which evaluated the safety and efficacy of pegcetacoplan in 1,258 patients.20 ACP was assessed in the GATHER1/GATHER2 trials.21,22
To be included in all trials, patients had to have a BCVA of 20/320 or better, no neovascularization or exudation in the study eye, and a total GA area between 2.5 and 17.5 mm2 via FAF. The primary endpoint was change in total GA lesion area on FAF. Although the inclusion criteria was the same, there were key differences in the study design. In GATHER1/GATHER2, only patients with noncenter point-involving GA in part within 1500 μm from the foveal center were included; patients with CNV in the fellow eye were excluded.21,22 For OAKS and DERBY, patients with GA lesions with and without subfoveal involvement were included, and CNV in the fellow eye was not exclusionary.20 These studies were conducted very differently.
ACP: GATHER1/GATHER2 Data
GATHER1 was a two-part trial. In part 1, patients were randomly assigned to ACP 1 mg or 2 mg or sham. In part 2, patients were randomly assigned to ACP 2 mg or 4 mg or sham. The primary endpoint was the mean rate of change in GA lesion size. The GATHER1 program was assessed over a total of 18 months.19
In GATHER2, patients were randomly assigned to ACP 2 mg (the FDA-approved dose) or sham. Patients were treated monthly, like they were in GATHER1, for the first 12 months. The primary endpoint was at 12 months, but the study was conducted through 24 months. In year 2, patients were rerandomized to ACP 2 mg monthly or every other month.20
Both GATHER1 and GATHER2 met their primary endpoints, meaning that the lesion growth rate was slowed in patients receiving monthly ACP versus sham. In GATHER2, patients who were dosed monthly through 2 years had a 14% reduction in the mean rate of GA growth at 2 years from baseline versus sham. Patients in the every-other-month group experienced a 19% mean reduction in GA growth rate at 2 years versus sham (Figure 11).23
A pooled analysis of GATHER1/GATHER2 showed that the early treatment effect of ACP 2 mg was observed by 6 months and increased over time through 18 months. There was an almost two-fold increase in treatment affect with ACP 2 mg versus sham, over time (Figure 12).24
A post hoc analysis of the GATHER1/GATHER2 trial showed that ACP treatment resulted in an overall 59% risk reduction in the rate of vision loss compared with sham at 12 months.25 This is the information patients want; will they lose vision? I think a 59% risk reduction is a reasonable, usable number because it’s an average of that whole cohort. This is the first time an interventional GA study has shown a relationship between GA growth and vision loss (Figure 13).
But it’s important to remember that this is a post hoc analysis. This is not the primary endpoint or even the secondary endpoint. These are tricky data.


Figure 11. 24-month analysis of the GATHER2 trial.

Figure 12. Difference in mean rate of GA growth from baseline compared to sham.24


Figure 13. GATHER1 and GATHER2 post hoc analysis results.25
ACP Adverse Events
For ACP, adverse reactions include subconjunctival hemorrhage (13%), increased intraocular pressure (9%), blurred vision (8%), CNV (7%), eye pain (4%), vitreous floaters (2%) and blepharitis (2%). There were no reports of intraocular inflammation in GATHER1 or GATHER2.21,22
Pegcetacoplan: OAKS, DERBY, and GALE Data
Pegcetacoplan was approved based on the phase 3 OAKS and DERBY trials, which assessed the root-mean-square of GA growth, the primary endpoint. Visual acuity was the secondary endpoint of both trials. OAKS and DERBY had two pegcetacoplan treatment arms, every month and every other month, compared with sham. At 12 months, OAKS met the primary endpoint, but DERBY did not.20 However, when pooled together, the 24-month data showed that pegcetacoplan slowed GA growth by 23% when injected monthly, and 22% when injected every other month. At 2 years, there was no difference in the average visual function between pegcetacoplan and sham.26
The GALE extension study of pegcetacoplan went out to year 3, further demonstrating that pegcetacoplan has increasing effects over time, with reduction of GA growth by 35% in the monthly group and 24% in the every-other-month group (Figure 14).27
The GALE extension study also showed that pegcetacoplan preserved visual function at 36 months (Figure 15), statistically significant in the monthly injection group only.28 In a prespecified microperimetry endpoint, patients developed fewer new scotomatous points with 36 months of both continuous monthly (P = .0156) and every-other-month (P = .1233) pegcetacoplan treatment compared with sham. Scotomatous points measure areas of the retina that have lost all light sensitivity and are no longer functioning.

Figure 14. OAKS, DERBY, and GALE data.27

Figure 15. GALE demonstrated that pegcetacoplan preserves visual function at 36 months.28
A recent post hoc analysis of OAKS assessed microperimetry endpoints at baseline and every 6 months until 24 months, using a 10-2 grid composed of 68 points with a 4-2 threshold strategy.29 The main outcome measures were the time to development of absolute scotomas in the 4 and 16 central macular points. The number of absolute scotomatous points and mean retinal sensitivity (dB) within the junctional zone extending to 250 μm on either side of autofluorescence-determined GA border was analyzed for change from baseline.
Monthly and every-other-month pegcetacoplan treatment delayed time to development of absolute scotomas of all 4 central macular points compared to sham at 24 months. Similarly, monthly and every-other-month treatment delayed time to development of absolute scotomas of all 16 central points (Figure 16). Across the junctional zone of GA, pegcetacoplan-treated eyes developed fewer absolute scotomatous points and experienced decreased loss of mean retinal sensitivity compared with sham at 24 months.29


Figure 16. Microperimetry analyses suggest central visual field benefit with pegcetacoplan.29
Pegcetacoplan’s effectiveness is even more pronounced on photoreceptor survival (OCT). Researchers quantified morphological changes of the photoreceptors and RPE layers under pegcetacoplan therapy using deep learning– based analysis of OCT images. They found a reduction of RPE loss growth by 22% and 20% in OAKS and 27% and 21% in DERBY for monthly and every-other-month treatment compared with sham, respectively, at 24 months (Figure 17).30 The photoreceptors were even more affected than the RPE loss, with a 53% and 46% reduction in OAKS and a 47% and 46% reduction in DERBY, for monthly and every-other-month treatment, respectively, at 24 months.
Figure 18 shows how pegcetacoplan shifts people from faster progression GA to slower progression GA. It’s a little bit of an extrapolation there, but the analyses demonstrated the consistent efficacy of pegcetacoplan across patient subgroups and with monthly and every-other-month dosing. The slowest progressing quartile consisted of a higher proportion of patients treated with pegcetacoplan than sham.31

Figure 17. How pegcetacoplan effects photoreceptors and RPE.30

Figure 18. Pegcetacoplan shifts GA from faster to slower-growing phenotypes.31
Pegcetacoplan Adverse Reactions
Adverse reactions for pegcetacoplan include ocular discomfort (13%), wet AMD (12%), vitreous floaters (10%), subconjunctival hemorrhage (8%), vitreous detachment (4%), retinal hemorrhage (4%), punctate keratitis (5%), posterior capsule opacification (4%), intraocular inflammation (4%), and increased intraocular pressure (2%).20,26
After reports of intraocular inflammation following pegcetacoplan treatment, the American Society of Retina Specialists (ASRS) Research and Safety in Therapeutics (ReST) Committee performed a retrospective review
of retinal vasculitis cases that were reported to ASRS.32 They concluded that the risk of vasculitis is small, with 14 eyes of 13 patients confirmed to have retinal vasculitis by review of imaging studies. Occlusive retinal vasculopathy was confirmed in 11 (79%) of eyes. All cases occurred after the first pegcetacoplan injection, with patients presenting a median of 10.5 days after treatment. The vasculitis involved the veins more than the arteries, and there were retinal hemorrhages all over. Pain and corneal edema are common presenting signs. At the most recent follow-up, 8 (57%) eyes had >3 line decrease in visual acuity, and 6 (43%) eyes had >6 line decrease in visual acuity from baseline at final follow-up, including 2 eyes that were enucleated. Six eyes (43%) developed signs of anterior segment neovascularization.32
Even though vasculitis occurs in a very small percentage, I still discuss this with every patient. It’s a real risk. That said, I do think there is a place for these medications.
A retrospective review from the Retinal Consultants of America, a nationwide group that includes more than 250 retina specialists, and Mid Atlantic Retina, helps put this risk into context as well as gives a real-world analysis of how we use these medications as retina specialists.33 The study assessed 6,525 patients treated with pegcetacoplan for a total of 32,080 injections over 14 months. The mean age of these patients was about 82 years old. Patients had a mean VA of 20/50 and were predominately female (67.2%). About 10% stopped therapy during the study period. There were 296 new cases of CNV, 29 cases of mild intraocular inflammation (0.4%), and 4 cases of retinal vasculitis (0.06% per patient risk).
It’s important to remember that the risk of endophthalmitis with any intravitreal injection is about 1 in 4,000. The risk of endophthalmitis with pegcetacoplan injection is about 1 in 10,000.34,35
Given these data, how do we educate patients? I tell patients about all their options, but I never push them into it. I think patients need to know what’s out there, but they need to understand that these treatments are not going to stop GA, cure GA, or reverse vision loss. These treatments are trying to slow the progression of GA. Patients need to know what they are signing up for: this is monthly or every-other-month treatment and likely forever. I think these are good medications, but they are not fantastic.
The current available treatments for GA are a great start, but we are hoping for better treatment options in the future. There are many ongoing clinical trials on additional medications that seem promising. All of our current available treatments slow disease progression but do not reverse it. This is why it is so important to identify GA patients early and refer them to retina specialists before advanced disease has taken their vision.
- Kaiser PK, Karpecki PM, Regillo CD, et al. Geographic Atrophy Management Consensus (GA-MAC): a Delphi panel study on identification, diagnosis and treatment. BMJ Open Ophthalmol. 2023;8(1).
- Virgili G, Michelessi M, Parodi MB, Bacherini D, Evans JR. Laser treatment of drusen to prevent progression to advanced age-related macular degeneration. Cochrane Database Syst Rev. 2015(10):Cd006537.
- Lindblad AS, Lloyd PC, Clemons TE, et al. Change in area of geographic atrophy in the Age-Related Eye Disease Study: AREDS report number 26. Arch Ophthalmol. 2009;127(9):1168-1174.
- Schmitz-Valckenberg S. The Journey of “Geographic Atrophy” through Past, Present, and Future. Ophthalmologica. 2017;237(1):11-20.
- IZERVAY Prescribing Information. Accessed February 21, 2025. https://www.astellas.com/us/system/files/izervay_pi.pdf
- Syfovre Prescribing Information. Accessed February 21, 2025. https://pi.apellis.com/files/PI_SYFOVRE.pdf
- Guymer RH, Rosenfeld PJ, Curcio CA, et al. Incomplete Retinal Pigment Epithelial and Outer Retinal Atrophy in Age-Related Macular Degeneration: Classification of Atrophy Meeting Report 4. Ophthalmology. 2020;127(3):394-409.
- Amarasekera S, Samanta A, Jhingan M, et al. Optical coherence tomography predictors of progression of non-exudative age-related macular degeneration to advanced atrophic and exudative disease. Graefes Arch Clin Exp Ophthalmol. 2022;260(3):737-746.
- Sadda SR, Guymer R, Holz FG, et al. Consensus Definition for Atrophy Associated with Age-Related Macular Degeneration on OCT: Classification of Atrophy Report 3. Ophthalmology. 2018;125(4):537-548.
- Keenan TD, Agrón E, Domalpally A, et al. Progression of Geographic Atrophy in Age-related Macular Degeneration: AREDS2 Report Number 16. Ophthalmology. 2018;125(12):1913-1928.
- Fleckenstein M, Mitchell P, Freund KB, et al. The Progression of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Ophthalmology. 2018;125(3):369-390.
- Sunness JS, Margalit E, Srikumaran D, et al. The long-term natural history of geographic atrophy from age-related macular degeneration: enlargement of atrophy and implications for interventional clinical trials. Ophthalmology. 2007;114(2):271-277.
- Chakravarthy U, Bailey CC, Johnston RL, et al. Characterizing Disease Burden and Progression of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Ophthalmology. 2018;125(6):842-849.
- Leng T, Schwartz J, Nimke D, et al. Dry Age-Related Macular Degeneration: Distribution of Visual Acuity and Progression Risk in a Large Registry. Ophthalmol Ther. 2023;12(1):325-340.
- Krogh Nielsen M, Hinnerskov JMV, Sørensen TL. Geographic atrophy - Signs, symptoms, and quality of life. Acta Ophthalmol. 2023;101(8):896-902.
- Bakri SJ, Amoaku WMK, Altman D, et al. The MOSAIC Study: A Mixed-Methods Study of the Clinical, Emotional, and Financial Burden of Geographic Atrophy Among Patients and Caregivers in the US. Clin Ophthalmol. 2024;18:2357-2368.
- Patel PJ, Ziemssen F, Ng E, et al. Burden of Illness in Geographic Atrophy: A Study of Vision-Related Quality of Life and Health Care Resource Use. Clin Ophthalmol. 2020;14:15-28.
- Bhattad PB, Pacifico L. Empowering Patients: Promoting Patient Education and Health Literacy. Cureus. 2022;14(7):e27336.
- Dahlin-Ivanoff S, Klepp KI, Sjöstrand J. Development of a health education programme for elderly with age-related macular degeneration: a focus group study. Patient Educ Couns. 1998;34(1):63-73.
- Heier JS, Lad EM, Holz FG, et al. Pegcetacoplan for the treatment of geographic atrophy secondary to age-related macular degeneration (OAKS and DERBY): two multicentre, randomised, double-masked, sham-controlled, phase 3 trials. Lancet. 2023;402(10411):1434-1448.
- Jaffe GJ, Westby K, Csaky KG, et al. C5 Inhibitor Avacincaptad Pegol for Geographic Atrophy Due to Age-Related Macular Degeneration: A Randomized Pivotal Phase 2/3 Trial. Ophthalmology. 2021;128(4):576-586.
- Loewenstein A, Tadavoni R. EURETINA Educational Debate 9: January Double Debate. Presented at: EURETINA. January 11, 2024.
- Crago SM. Angiogenesis 2024: Expanded efficacy data from the GATHER 2 trial. Ophthalmology Times Europe. February 3, 2024. Accessed February 21, 2025. https://europe.ophthalmologytimes.com/view/angiogenesis-2024-expanded-efficacy-data-from-the-gather-2-trial
- Khanani AM, Patel SS, Staurenghi G, et al. Efficacy and safety of avacincaptad pegol in patients with geographic atrophy (GATHER2): 12-month results from a randomised, double-masked, phase 3 trial. Lancet. 2023;402(10411):1449-1458.
- Danzig CJ, Khanani AM, Kaiser PK, et al. Vision Loss Reduction with Avacincaptad Pegol for Geographic Atrophy: A 12-Month Post Hoc Analysis of the GATHER1 and GATHER2 Trials. Ophthalmol Retina. 2024;8(11):1052-1060.
- Fu DJ, Bagga P, Naik G, et al. Pegcetacoplan Treatment and Consensus Features of Geographic Atrophy Over 24 Months. JAMA Ophthalmol. 2024;142(6):548-558.
- Wykoff CC. Long-term efficacy and safety of pegcetacoplan from the GALE open-label extension of the phase 3 OAKS and DERBY trials. AAO; 2023; San Francisco.
- Apellis Pharmaceuticals I. SYFOVRE® (pegcetacoplan injection) Preserved Visual Function at 36 Months in GALE Extension Study in Geographic Atrophy (GA). Accessed March 14, 2025. https://investors.apellis.com/news-releases/news-release-details/syfovrer-pegcetacoplaninjection-preserved-visual-function-36
- Chakravarthy U, Schwartz R, Guymer RH, et al. Visual Function Benefit After Treatment With Pegcetacoplan: Microperimetry Analysis From The Phase 3 Oaks Trial: Microperimetry: Visual Function Benefit With Pegcetacoplan. Am J Ophthalmol. 2025.
- Schmidt-Erfurth U, Mai J, Reiter GS, et al. Disease Activity and Therapeutic Response to Pegcetacoplan for Geographic Atrophy Identified by Deep Learning-Based Analysis of OCT. Ophthalmology. 2025;132(2):181-193.
- Chiang A, Bliss C, Ribeiro R. Assessment of geographic atrophy (GA) lesion progression in the phase 3 OAKS and DERBY trials. IOVS. 2023;64(8):986-986.
- ASRS. New ReST Committee Report Published in JVRD Summarizes Analysis of Reported Cases of Retinal Vasculitis Following Syfovre Injection.
- Minaker S. Real-world practice patterns and adverse events of pegcetacoplan injection for geographic atrophy. ASCRS 2024.
- McCannel CA. Meta-analysis of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents: causative organisms and possible prevention strategies. Retina. 2011;31(4):654-661.
- Englander M, Chen TC, Paschalis EI, Miller JW, Kim IK. Intravitreal injections at the Massachusetts Eye and Ear Infirmary: analysis of treatment indications and postinjection endophthalmitis rates. Br J Ophthalmol. 2013;97(4):460-465.




































