Topical Therapies
Novel topical agents for the treatment of early- to moderate-stage glaucoma are emerging and can play a role in clinical practice. Omidenepag isopropyl (OMDI) 0.002% is approved for treatment of open-angle glaucoma and ocular hypertension.1 Topical administration is once daily in the evening to the affected eye. This less frequent dosing due to longer duration of action is one advantage of OMDI over other topical medications.
OMDI, once it penetrates the cornea and is hydrolyzed into its active form, is a selective prostaglandin EP2 receptor agonist.2 The prostaglandin EP2 receptor is a transmembrane G protein-coupled receptor that increases cyclic AMP levels to facilitate aqueous humor outflow through both the trabecular and the uveoscleral pathways. In the AYAME trial, OMDI was noninferior in reducing intraocular pressure (IOP; least squares mean -5.93 ± 0.23 mmHg) compared to latanoprost 0.005% at 4 weeks (-6.56 ± 0.22 mm Hg; 95% confidence interval between groups: 0.01-1.26).3 OMDI is also associated with fewer long-term prostaglandin-associated periorbitopathy (PAP) complications, such as fat atrophy and hyperpigmentation, compared to prostaglandin F receptor agonists (Figure 1).2
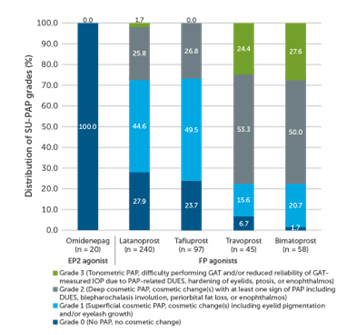
Figure 1. Prostaglandin-associated periorbitopathy complications.2
Netarsudil/latanoprost is a fixed-dose combination medication approved for the treatment of open-angle glaucoma and ocular hypertension.4 One advantage of netarsudil/latanoprost is its longer duration of action; administration is once daily in the evening to the affected eye. Netarsudil is a rho-kinase inhibitor that increases aqueous humor outflow through the conventional pathways while also decreasing episcleral venous pressure and oxidative stress.5 When combined with the selective prostaglandin F receptor agonist, latanoprost there is an additive IOP-lowering effect.
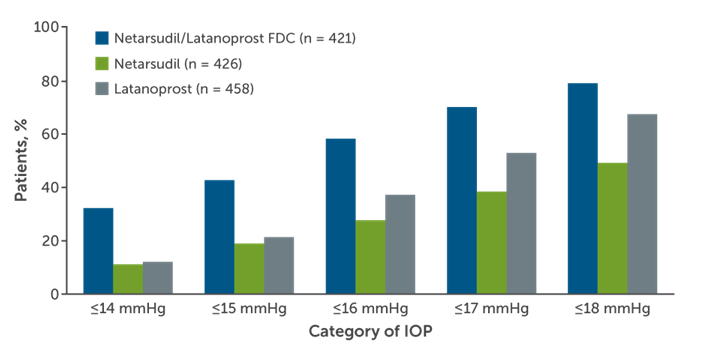
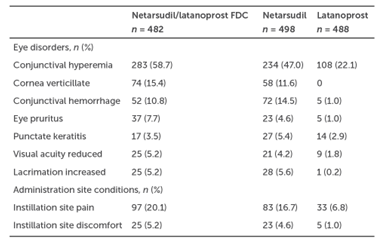
Efficacy of netarsudil/latanoprost was evaluated in a pooled analysis of the MERCURY-1 and -2 phase 3 superiority studies comparing the agent to netarsudil or latanoprost monotherapy.6 Combination therapy produced IOP-lowering effects that were statistically in excess of either netarsudil or latanoprost monotherapy (Figure 2). A common adverse effect of combination netarsudil/latanoprost is conjunctival hyperemia in excess of what was observed with latanoprost or netarsudil monotherapy (Table 1). Other notable side effects include subconjunctival hemorrhage, corneal verticillata, tearing, instillation site pain, erythema, and eyelid erythema.6
A
B
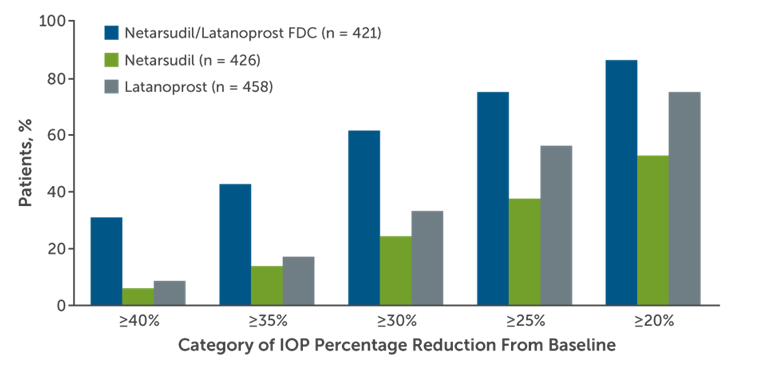
Figure 2. Percentages of MERCURY-1 and MERCURY-2 patients reaching prespecified (A) mean diurnal IOP targets and (B) categorical IOP percentage reductions from baseline in mean diurnal IOP at month 3.6
Table 1. Ocular Adverse Events in at Least 5% of Patients6
Brimonidine tartrate ophthalmic suspension is a new formulation of brimonidine using a proprietary resin microparticle complex drug delivery system.7,8 According to the manufacturer, a phase 3 equivalence trial that compared once-daily brimonidine tartrate suspension 0.35% to brimonidine tartrate solution 0.1% administered 3 times a day met its prespecified primary endpoint of noninferiority at 12 weeks (degree of IOP lowering has not yet been publicly reported[SW1] ).8 A decision is pending by the United States Food and Drug Administration regarding the use of brimonidine tartrate suspension for the treatment of open-angle glaucoma and ocular hypertension.9
Patient Cases: Topical Therapies
Adherence is one of the biggest challenges faced by patients with glaucoma. Let's consider a case where treatment selection can help the patient stay on therapy, control IOP, and preserve vision. An 85-year-old woman is being treated for low-tension glaucoma. Her baseline IOP averages 18 mmHg in both eyes. She has mild visual field loss and is currently using latanoprost for intraocular pressure control. She also has early Alzheimer’s disease and relies on her son to administer the drops after he gets back from work each night. She recently received selective laser trabeculoplasty (SLT), which was unfortunately ineffective.
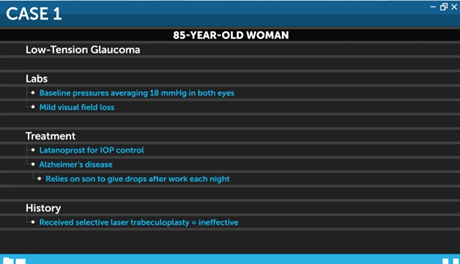
For this patient, combination netarsudil latanoprost would do well in managing her IOP. As previously noted, fixed-dose combination netarsudil latanoprost was superior to monotherapy of either netarsudil or latanoprost at 12 weeks.6 In particular, the number of patients achieving at least 30% IOP reduction after combination therapy was approximately double that of latanoprost monotherapy (Figure 2B). Given the limitations faced by this patient, combination therapy is likely to provide additional, combination therapy is likely to provide additional IOP control without needling to increase the frequency of medication administration. However, a discussion with the patient and caretaker is needed about ocular side effects, including conjunctival hyperemia and corneal verticillata, which are more common than with latanoprost monotherapy.
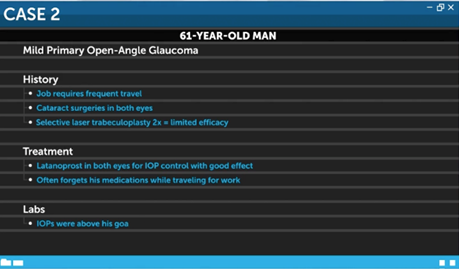
Next, let’s consider the case of a 61-year-old man who is a business executive with mild primary open-angle glaucoma. His job requires frequent travel. He has had cataract surgery in both eyes and SLT twice with limited efficacy. He is currently using latanoprost in both eyes for IOP control with good effect. However, he often forgets his medications while traveling for work. His IOP was above goal at his last visit. Also, he has noticed more ocular irritation and redness around the eye. Is there something that can be done to improve his IOP control?
Durysta is a biodegradable drug-eluting implant that contains 10 mg of bimatoprost. The implant is injected into the interior chamber. It has been shown to be noninferior to topical timolol after 12 weeks of treatment with durable efficacy even after a single injection.10,11
Patient selection for Durysta is important and should be assessed for each individual patient. The implant is indicated for IOP control in patients with open-angle glaucoma and ocular hypertension, which applies to this patient.12 Advantages of the implant include a single treatment with continuous drug delivery that does not rely on good compliance. Drug levels stemming from the implant on the ocular surface and periocular tissue have been found to be decreased compared to those after bimatoprost application, which may also decrease ocular surface and periocular irritation in this patient.13 Finally, Durysta is contraindicated in patients at risk for corneal endothelial cell loss, with narrow angles, disruption of the posterior lens capsule, a history of intraocular inflammation or macular edema, and an allergy to prostaglandin.12 None of these contraindications apply to this patient.
In a patient like this one with mild to moderate glaucoma, in whom intraocular pressure is well controlled on prostaglandin analogs, the bimatoprost intraocular implant is a good treatment alternative. One thing that should be discussed with the patient is that the implant is currently only approved for a one-time use. This means that repeat injections would incur out-of-pocket costs for the patient. The patient would also be at increased risk of endothelial cell loss.
Patient Cases: MIGS
MIGS devices are appropriate treatment options for many patients but require an individualized approach. The first case is a 75-year-old man with mild glaucoma and a visually significant cataract. He has thinning on the inferior rim of his optic nerve and a mild nasal step on visual field testing with a mean deviation of -3.5 dB. His IOP is 20 mmHg while using 2 antiglaucoma medications. The goal is to reduce IOP to 17 mmHg while using 1 antiglaucoma medication. He has had a prior SLT, to which he had a good response. Overall, he has been on glaucoma drops for about 4 years. He is also on blood thinners for treatment of atrial fibrillation. On examination, he has wide open angles with well-defined structures and landmarks. Finally, he is relatively active and exercises on a regular basis.
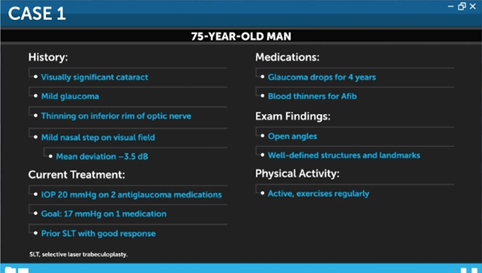
Given this patient’s need for blood thinners, his stage of glaucoma, and his active lifestyle, Dr. Grover chose to implant a Hydrus stent at the time of cataract surgery. He selected the stent as opposed to a goniotomy or trabeculotomy due to the high risk of recurrent hyphema. This patient’s case went well. During the surgery Dr. Grover was able to visualize the Hydrus implant in the canal for the entirety of its length. Postoperatively, the patient experienced a very fast visual and physical recovery. He did not have a postoperative hyphema. He was treated with antibiotics and steroids, as Dr. Grover would routinely treat for cataract surgery, with the exception that the patient continued topical steroids for an additional week. One year after the surgery, the patient’s IOP is well controlled, in the mid-teens, and he uses 1 prostaglandin drop at night.
The second case is a an 82-year-old woman with moderate to advanced glaucoma. She has previously undergone cataract surgery and goniotomy a few years ago, but her IOP has been slowly increasing. The goal is to reduce her IOP to less than 15 mmHg on as few medications as possible. Her IOP is currently 18 mmHg on 4 agents. Also, there has been a slow decline in her nerve fiber layer as visualized on optical coherence tomography over the past 2 years. The patient’s family says they are concerned that her medication compliance has declined lately.
The patient reports that her eyes are a little red and irritated, and the glaucoma drops are causing them to dry out. She has definitive glaucoma on visual field testing with a mean deviation of -8 dB. Additionally, she has a dense nasal step and a mild inferior arcuate defect. She has had 2 prior SLTs and has undergone phacoemulsification with goniotomy. He conjunctiva superiorly is very mobile.
Given the goal of an IOP in the low teens and to reduce her use of drops as much as possible, Dr. Grover opted for an ab interno, close conjunctival XEN-45 gel stent. He used 40 μg of mytomycin C. He used the posterior sweep of Tenon’s capsule (POST) technique, where after implanting the XEN stent, he swept away the Tenon’s with a microshunt spatula, essentially performing primary needling. The patient tolerated the procedure well. Given her prior goniotomy, she had a microhyphema at postoperative week 1, but it resolved quickly. On postoperative day 1, her IOP was 7 mmHg, her anterior chamber was formed and deep, and her vision was unchanged. She was treated with topical antibiotic and steroids, similar to what would be done for a cataract surgery. However, her steroids were tapered over a 5-6 week period. After 10-14 days, she was able to return to normal activities. It has now been 18 months since her surgery. Her IOP is 12 mmHg on 1 drop of timolol in the morning. Shas has a diffuse low bleb that is mildly vascularized.
These 2 cases exemplify how MIGS procedures can be tailored to the patient’s lifestyle, their disease state, their comorbidities, their blood thinner status, and their activity level. It’s an exciting time to be a glaucoma doctor. Just 20-30 years ago the only options available were trabeculectomy and tubes. Today, there are a lot of treatment options with greater safety that allow doctors to intervene in effective ways to preserve vision.
References
- Omidenepag isopropyl ophthalmic solution. Package insert. Santen Incorporated; 2022.
- Matsuo M, Matsuoka Y, Tanito M. Efficacy and patient tolerability of omidenepag isopropyl in the treatment of glaucoma and ocular hypertension. Clin Ophthalmol. 2022;16:1261-1279.
- Aihara M, Lu F, Kawata H, Iwata A, Odani-Kawabata N, Shams NK. Omidenepag isopropyl versus latanoprost in primary open-angle glaucoma and ocular hypertension: the phase 3 AYAME study. Am J Ophthalmol. 2020;220:53-63.
- Netarsudil and latanoprost ophthalmic solution. Package insert. Aerie Pharmaceuticals, Inc.; 2020.
- Lin CW, Sherman B, Moore LA, et al. Discovery and preclinical development of netarsudil, a novel ocular hypotensive agent for the treatment of glaucoma. J Ocul Pharmacol Ther. 2018;34(1-2):40-51.
- Asrani S, Bacharach J, Holland E, et al. Fixed-dose combination of netarsudil and latanoprost in ocular hypertension and open-angle glaucoma: pooled efficacy/safety analysis of phase 3 MERCURY-1 and -2. Adv Ther. 2020;37(4):1620-1631.
- Evaluation of safety and efficacy of PDP-716. ClinicalTrials.gov identifier: NCT03450629. Updated February 15, 2022. https://clinicaltrials.gov/ct2/show/NCT03450629
- SPARC declares positive results from phase 3 clinical trial of PDP-716 to treat open-angle glaucoma or ocular hypertension. EP News Bureau. Published May 14, 2021. Accessed May 9, 2022. https://www.expresspharma.in/sparc-declares-positive-results-from-phase-3-clinical-trial-of-pdp-716-to-treat-open-angle-glaucoma-or-ocular-hypertension/
- Hutton D. Visiox Pharma submits NDA seeking approval for first once-daily brimonidine for glaucoma. Ophthalmology Times. Published October 6, 2022. Accessed May 9, 2023. https://www.ophthalmologytimes.com/view/visiox-pharma-submits-nda-seeking-approval-for-first-once-daily-brimonidine-for-glaucoma
- Medeiros FA, Walters TR, Kolko M, et al. Phase 3, randomized, 20-month study of bimatoprost implant in open-angle glaucoma and ocular hypertension (ARTEMIS 1). Ophthalmology. 2020;127(12):1627-1641.
- Bacharach J, Tatham A, Ferguson G, et al. Phase 3, randomized, 20-month study of the efficacy and safety of bimatoprost implant in patients with open-angle glaucoma and ocular hypertension (ARTEMIS 2). Drugs. 2021;81(17):2017-2033.
- Bimatoprost intracameral implant. Package insert. Allergan USA, Inc.; 2020.
- Seal JR, Robinson MR, Burke J, Bejanian M, Coote M, Attar M. Intracameral sustained-release bimatoprost implant delivers bimatoprost to target tissues with reduced drug exposure to off-target tissues. J Ocul Pharmacol Ther. 2019;35(1):50-57.












 In support of improving patient care, Global Learning Collaborative (GLC) is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC) to provide continuing education for the healthcare team.
In support of improving patient care, Global Learning Collaborative (GLC) is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC) to provide continuing education for the healthcare team.













Facebook Comments