Omidenepag Isopropyl (OMDI)
OMDI is a novel prostaglandin E2 receptor agonist that increases aqueous outflow through the trabecular and uveoscleral pathways. The AYAME study was a phase 3, randomized noninferiority trial that evaluated the efficacy and safety of OMDI in Japanese patients with primary open-angle glaucoma (POAG).1 The purpose of the study was to compare the intraocular pressure (IOP)-lowering effects of OMDI 0.002% to latanoprost 0.005% at dose of one drop once daily at night for 4 weeks. Eligible patients were 20 years or older with a diagnosis of bilateral POAG or ocular hypertension with a visual acuity of 20/100 on a Snellen chart. Those with advanced visual filed loss, recent ocular surgery, a history of ocular inflammation and retinal pathology, or conditions interfering with accurate Goldmann applanation tonometry were excluded. After a washout period of 1-4 weeks, a total of 190 patients were randomized to either OMDI or latanoprost, with 89 completing the study in the OMDI group and 94 completing the study in the latanoprost group (Figure 1). IOP was measured at 9:00 AM, 1:00 PM, and 5:00 PM at weeks 1, 2, and 4. The primary endpoint was the change from baseline in mean diurnal IOP at week 4. The noninferiority margin for OMDI compared to latanoprost was 1.5 mmHg.1
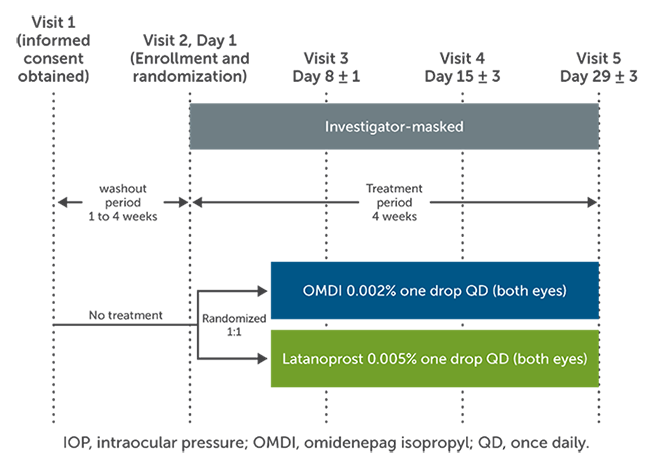
Figure 1. AYAME study design.1
Baseline characteristics were comparable between the 2 study groups.1 At week 4, least squares mean reduction IOP for OMDI, averaging 5.93, plus or minus a standard error of 0.23 mmHg, was found to be noninferior to that of latanoprost, which averaged 6.56, plus or minus a standard error of 0.22 mmHg. The treatment difference between the 2 groups was statistically, significant (P = 0.0477) but it is questionable whether a 0.63 mmHg difference is truly clinically significant. No serious adverse events, as determined by the investigators, were observed in either group. The incidence of adverse event was higher in the OMDI group than that in the latanoprost group. The most common ocular adverse events were conjunctival hyperemia (24.5%), corneal thickening (11.7%), and photophobia (4.3%), occurring in all cases more frequently in the OMDI group. In summary, after 4 weeks of treatment, OMDI was shown to be noninferior to latanoprost for IOP reduction in patients with ocular hypertension or POAG while exhibiting good tolerability.1
The RENGE and FUJI studies are two other phase 3 trials conducted in Japan.2,3 Results from those trials support the finding of the AYAME study and further suggest that OMDI 0.002% exhibits long-term efficacy whether alone or in combination with timolol 0.5%. Additionally, OMDI may be efficacious in non- or poor responders to latanoprost. Notably, a significant percentage of pseudophakic patients in the RENGE study exhibited macular edema after 52 weeks of treatment.2 Longer-term efficacy and safety data are needed; however, OMDI holds promise as a novel IOP-lowering agent for treating glaucoma.
Netarsudil/Latanoprost
Combination netarsudil/latanoprost may be a helpful option for glaucoma patients who require multiple topical agents. Netarsudil is a rho-kinase inhibitor that is thought to increase aqueous humor outflow through the conventional pathway. Latanoprost is a prostaglandin analog that reduces intraocular pressure by increasing uveoscleral outflow.4
MERCURY-1 and MERCURY-2 were phase 3 superiority studies comparing a fixed-dose combination of netarsudil 0.02% and latanoprost 0.005% to monotherapies of either netarsudil or latanoprost.5 Only adult patients with a diagnosis of open-angle glaucoma (OAG) and ocular hypertension were included in the studies. Patients with visual acuity worse than 20/200 on the Snellen chart and a history of ocular or systemic conditions thought likely to bias study results were excluded. After washout, IOP in both eyes had to be between 20 mmHg and 36 mmHg during the 8:00 AM visit and between 17 mmHg and 36 mmHg during the 10:00 AM and the 4:00 PM visits to be included. Qualifying patients were randomized to receive either netarsudil/latanoprost, single-agent netarsudil, or single-agent latanoprost to be applied daily in the evening, between 8 PM and 10 PM.5
The primary endpoint consisted of comparing mean IOP at 3 time points during the day at weeks 2, 6, and 12. Secondary endpoints compared mean diurnal IOP at each posttreatment visit and examining the proportion of patients achieving prespecified levels of mean diurnal IOP and percent change in diurnal IOP.5
A combined total of 1,468 patients were enrolled the MERCURY-1 and MERCURY-2 studies and 1,195 patients (81.4%) completed the studies.5 MERCURY-1 patients were treated for 12 months, while MERCURY-2 patients were treated for 3 months. Baseline demographic characteristics in the pooled populations were similar across all treatment groups. Results showed that the netarsudil/latanoprost fixed-dose combination met the criteria for superiority compared with netarsudil or latanoprost alone at all time points (P < 0.0001; Figure 2). Fixed-dose combination therapy surpassed monotherapy with respect to achieving all levels of prespecified IOP goals following treatment and percent IOP reduction from baseline (Figure 3). The number of patients who achieved at least 30% IOP reduction from baseline after combination therapy was approximately double that of latanoprost monotherapy.5
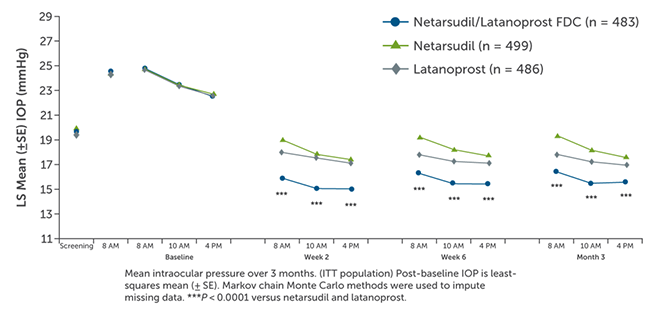
Figure 2. Pooled efficacy analysis of netarsudil/latanoprost vs monotherapy.5
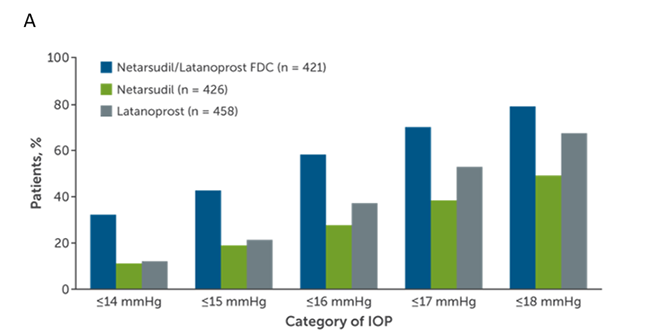
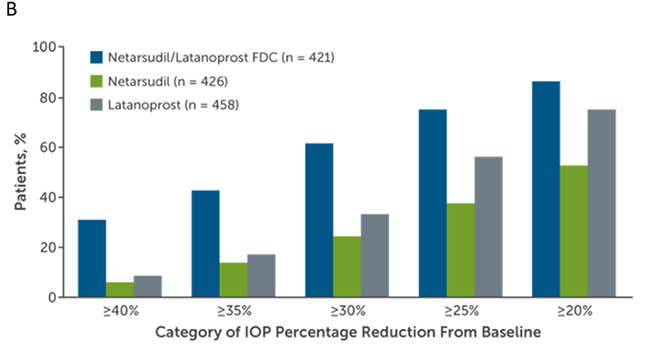
Figure 3. Percentages of MERCURY-1 and MERCURY-2 patients reaching prespecified (A) mean diurnal IOP targets and (B) categorical IOP percentage reductions from baseline in mean diurnal IOP at month 3.5
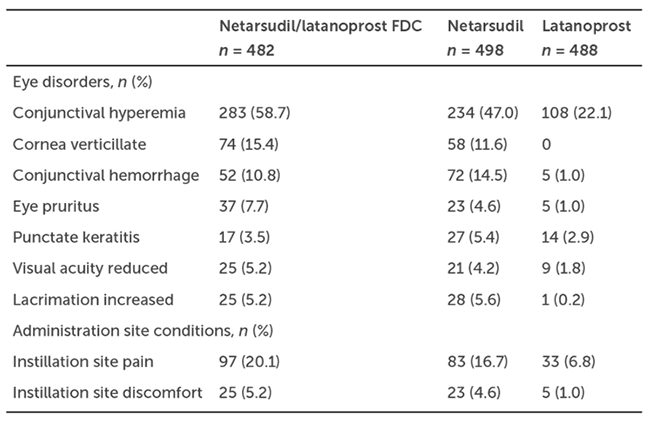
Treatment-related adverse events and ocular adverse events were highest following combination therapy (Table 1).5 The most common reason for discontinuation of fixed-dose combination netarsudil/latanoprost was adverse events associated with netarsudil. The most common ocular adverse event was conjunctival hyperemia, resulting in a higher rate of discontinuation of therapy in the combination and the netarsudil groups compared to the latanoprost group. Other ocular adverse events of note were corneal verticillata in the combination and netarsudil groups, conjunctival hemorrhage, tearing, decreased acuity, and instillation site pain and discomfort.5
Table 1. Ocular Adverse Events in at Least 5% of MERCURY-1 and MERCURY-2 Patients5
In summary, netarsudil/latanoprost combination therapy was superior to either netarsudil or latanoprost monotherapy during and after 12 weeks of treatment. Ocular side effects, while generally mild, were more frequent than latanoprost monotherapy and resulted in higher rates of treatment discontinuation. Given the fact that single-agent therapies are often insufficient to achieve desired IOPs, a once daily combination therapy like netarsudil/latanoprost is a good option for patients who are tolerant of both agents without increasing the frequency of medication administration.
Bimatoprost Implant
The bimatoprost implant is a novel treatment option providing durable IOP-lowering effects. The efficacy and safety of 2 doses of a bimatoprost biodegradable drug-eluting implant were compared to topical timolol in two 20-month, phase 3, randomized clinical trials. The ARTEMIS studies enrolled adults with OAG and ocular hypertension who were known responders to topical beta-blockers and prostaglandin analogs.6,7
Additional inclusion criteria were baseline IOPs between 22 mmHg and 32 mmHg at 8:00 AM and between 19 mmHg and 32 mmHg 2 hours thereafter, when topical timolol might be expected to reach peak efficacy. Also, patients were required to have a central corneal endothelial density ³1,800 cells per mm2.6,7
Participants were randomized to implants containing either 10 μg or 15 μg of bimatoprost or to topical timolol, 0.5%, administered twice daily.6 Implants were injected through the cornea and into the anterior chamber on day 1, then again on weeks 16 and 32. Primary endpoints were IOP and IOP changes from baseline at weeks 2, 6, and 12. Long-term efficacy and safety profiles, after repeated administration, were evaluated through month 20.6,7
By week 12, patients randomized to both strengths of the bimatoprost implant achieved IOP reductions from approximately 24 mmHg to 17 mmHg, representing a 30% reduction from baseline (Figure 4).6 In both trials, both strengths of the bimatoprost implant met predefined criteria of noninferiority compared to timolol. The implant demonstrated statistical noninferiority of timolol with respect to IOP lowering in the 12 weeks after the second and the third injections. A Kaplan-Meier survival analysis estimated a probability of not requiring additional treatment for 1 year after the last implant injection to be in the range of 70%-75% for participants.6,7
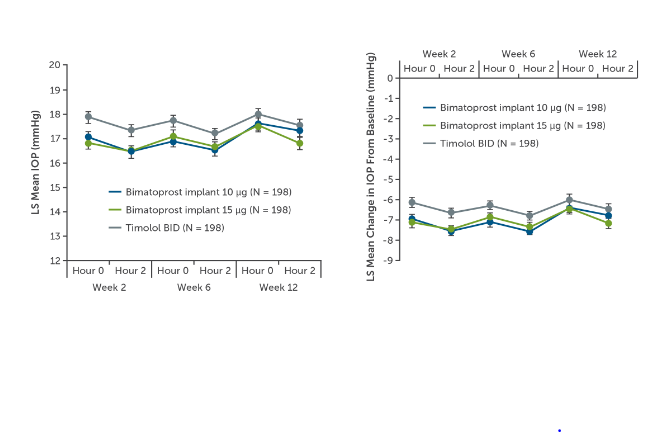
Figure 4. ARTEMIS primary endpoints of (A) mean IOP and (B) mean change in IOP from baseline through week 12.6
The most common treatment-emergent adverse events were conjunctival hyperemia, ocular irritation, foreign body sensation, and conjunctival hemorrhages, most of which were thought to be related to the injection protocol and the use of Povidone-iodine antiseptic on the ocular surface.6 Corneal endothelial cell loss, edema, and iritis were more frequent following bimatoprost implant administration compared to topical timolol. In particular, corneal endothelial cell density showed time-dependent loss in study eyes in the bimatoprost implant groups, with greater loss in the 15-μg compared to the 10-μg group. This difference was thought to be in part related to the larger size of the 15-μg implant.6,7
Based on trial results, the bimatoprost implant 10 μg is approved for a single intracameral administration for IOP control in patients with OAG or ocular hypertension.8 The implant was shown to be noninferior to timolol with respect to IOP lowering after 12 weeks, and endothelial cell loss was reported to be in the range of 5% after repeat injections in 20 months of follow-up.6,7 Use of the implant is contraindicated in individuals with active or suspected ocular or periocular infections, corneal endothelial cell dystrophy, prior corneal transplantation, absent or ruptured posterior lens capsules, and in patients with a history of hypersensitivity to bimatoprost.8 In summary, the bimatoprost implant is a good option for decreasing the burden of daily medication administration and has the potential to minimize ocular surface adverse effects in prostaglandin responders.
References
- Aihara M, Lu F, Kawata H, Iwata A, Odani-Kawabata N, Shams NK. Omidenepag isopropyl versus latanoprost in primary open-angle glaucoma and ocular hypertension: the phase 3 AYAME study. Am J Ophthalmol. 2020;220:53-63.
- Aihara M, Lu F, Kawata H, Iwata A, Odani-Kawabata N. Twelve-month efficacy and safety of omidenepag isopropyl, a selective EP2 agonist, in open-angle glaucoma and ocular hypertension: the RENGE study. Jpn J Ophthalmol. 2021;65(6):810-819.
- Aihara M, Ropo A, Lu F, et al. Intraocular pressure-lowering effect of omidenepag isopropyl in latanoprost non-/low-responder patients with primary open-angle glaucoma or ocular hypertension: the FUJI study. Jpn J Ophthalmol. 2020;64(4):398-406.
- Lin CW, Sherman B, Moore LA, et al. Discovery and preclinical development of netarsudil, a novel ocular hypotensive agent for the treatment of glaucoma. J Ocul Pharmacol Ther. 2018;34(1-2):40-51.
- Asrani S, Bacharach J, Holland E, et al. Fixed-dose combination of netarsudil and latanoprost in ocular hypertension and open-angle glaucoma: pooled efficacy/safety analysis of phase 3 MERCURY-1 and -2. Adv Ther. 2020;37(4):1620-1631.
- Medeiros FA, Walters TR, Kolko M, et al. Phase 3, randomized, 20-month study of bimatoprost implant in open-angle glaucoma and ocular hypertension (ARTEMIS 1). Ophthalmology. 2020;127(12):1627-1641.
- Bacharach J, Tatham A, Ferguson G, et al. Phase 3, randomized, 20-month study of the efficacy and safety of bimatoprost implant in patients with open-angle glaucoma and ocular hypertension (ARTEMIS 2). Drugs. 2021;81(17):2017-2033.
- Bimatoprost intracameral implant. Package insert. Allergan USA, Inc.; 2020.
























Facebook Comments