Bleb-Forming MIGS
XEN Gel Stent
The XEN Gel Stent (XEN) is a mircoshunt with an inner diameter of 45 µm (Figure 1).1 Among the many studies published on the outcomes of patients with the stent, the study by Reitsamer et al is notable with 3 years of outcomes.2 This study was a retrospective study based in Europe. At baseline eyes has a mean intraocular pressure (IOP) of 20.7 mmHg on 2.5 medications (n = 163), and at 3 years, study eyes had a mean IOP of 13.9 mmHg on 1.1 medications (n = 76).2
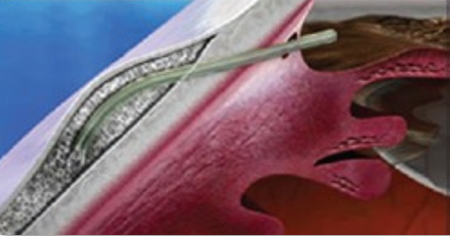
Figure 1. XEN Gel Stent.1
The majority of the patients in the Reitsamer et al study were of European descent, and researchers tended to use lower amounts of mitomycin C (10-20 μg). The needling rate was higher at 43%, whereas needling rates of 20% were typically observed in other studies. The success rate with XEN was 65.8% with slightly greater success rates in the XEN alone group versus the XEN + phacoemulsification group. Additional glaucoma surgery was required in 12.3% of cases, but there were no safety concerns overall.2
The XEN can be implanted with a variety of techniques: ab interno, ab externo, with or without conjunctival peritomy. When considering the studies of XEN implantation with those different techniques, the outcomes are very similar. This shows that surgeons should use the technique they are most comfortable with when implanting XEN.
PreserFlo MicroShunt
Another MIGS device is the PreserFlo MicroShunt which has an inner diameter of 70 µm and a length of 8.5 mm (Figure 2).3 The device is approved in Europe and Canada, but not in the United States. IN005 was the completed US Food and Drug Administration (FDA) trial of the PreserFlo MicroShunt and was a prospective, randomized, multicenter, interventional trial that showed no significant safety concerns.4 Both groups showed a significant decrease in IOP and a decrease in glaucoma drop dependence (Figure 3). However, the probability of success was lower in the PreserFlo group compared to the trabeculectomy group at 1 year (53.9% vs 72.7%, respectively). At the same time, hypotony was higher with the trabeculectomy group than in the PreserFlo group (49.6% vs 28.9%, respectively; P < 0.01).4
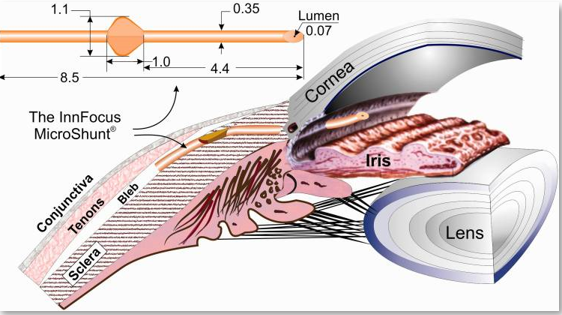
Figure 2. PreserFlo MicroShunt.3
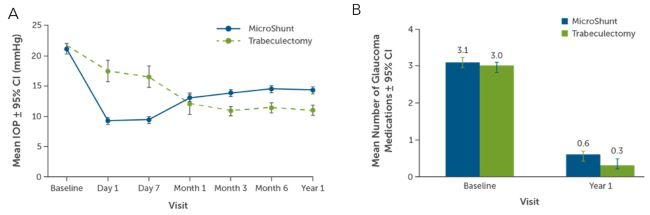
Figure 3. Efficacy of PreserFlo as measured by (A) mean IOP and (B) mean number of glaucoma medications.4
According to Dr. Grover, PreserFlo would likely have been approved had the study been noncomparative. However, IN005 was a comparative study, and it is difficult for current MIGS devices to reduce IOP more than trabeculectomy. Dr. Grover believes that one of the reasons PreserFlo was not approved was because PreserFlo was not superior to trabeculectomy. Trabeculectomy has the benefit of achieving lower IOPs, whereas microshunts protect against hypotony. Also, microshunts typically require higher doses of mitomycin C,a which was seen in the studies published by groups in Europe and Canada. These higher doses of mitomycin C were unlikely to be used in an FDA-approved trial.
Microshunts and the techniques for delivering microshunts will continue to evolve and improve in the future. Dr. Gover envisions that microshunts will likely replace trabeculectomy sometime in the next couple of years if there is continued improvement.
Blebless MIGS
iStent
When discussing the iStent, there is the iStent inject W and iStent infinite. Both stent configurations are the same, which is a flange of 360 µm, an inner diameter of 80 µm, and 4 outflow pathways on the side (Figure 4). The difference is in the delivery platform and indication. The iStent inject W is 2 stents on a preloaded injector and is indicated to be used in combination with cataract surgery. The iStent infinite is 3 preloaded stents with an infinite number of delivery attempts. This is the first FDA-approved device for standalone trabecular bypass that is not dependent on cataract surgery or disease state.
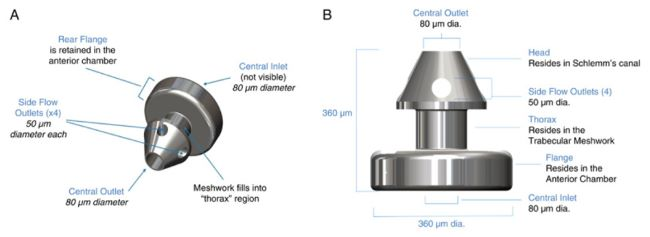
Figure 4. iStent.5
In a clinical trial with iStent inject, 75.8% of treated eyes (n = 380) vs 61.9% of eyes without stent implantation (control) had a 20% reduction from baseline IOP at 2 years (Figure 5).6 Patients in the study were on 1 to 4 ocular hypotensive medications at the time of study screening. After treatment, 63.2% of eyes were free of medications versus 50% of control eyes at 23 months.6 After 5 years, there were no significant concerns about endothelial cell loss with the iStent inject.7
a Only use doses of mitomycin C that are proven safe and effective.
The clinical trial evaluating the efficacy of iStent infinite was performed in patients with more treatment refractory glaucoma.5 At baseline, 72 eyes in 72 patients had a mean preoperative IOP of 23.4 mmHg on 3.1 medications. Among those, 61 eyes had failed prior surgery, and the remaining 11 eyes were uncontrolled on maximum medical therapy. A total of 76.1% of the enrolled patients met the primary endpoint, which was a ³20% reduction in mean IOP on the same or fewer medications. Additionally, 53% of eye had a ³30% reduction in IOP with any additional surgical interventions. The safety profile was favorable with no real concerns. These outcomes are impressive for a refractory group of patients.
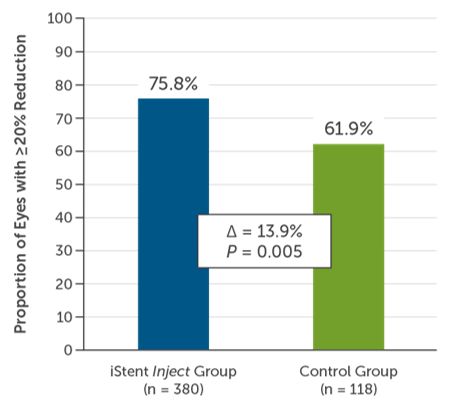
Figure 5. Efficacy of iStent inject after 2 years.
Hydrus
Hydrus is an 8-mm nitinol implant (Figure 6).8 The HORIZON trial consisted of 369 eyes randomized to the Hydrus microshunt and 187 eyes randomized to cataract surgery only.9 At 5 years with 80% of patients completing the study, the Hydrus group had a higher proportion of eyes with an IOP £18 mmHg and more eyes with an IOP reduction of ³20% without medications compared to the cataract group (Figure 7). Eyes in the Hydrus groups were also on fewer medications versus the control group (Figure 8). Finally, the cumulative risk of incisional glaucoma surgery was lower in the Hydrus group compared to cataract surgery, and there were no significant differences in the rate of endothelial cells loss (P = 0.261). Overall, the addition of the Hydrus implant to cataract surgery was safe in lowering medication use and IOP and reduced the need for incisional glaucoma surgery.9
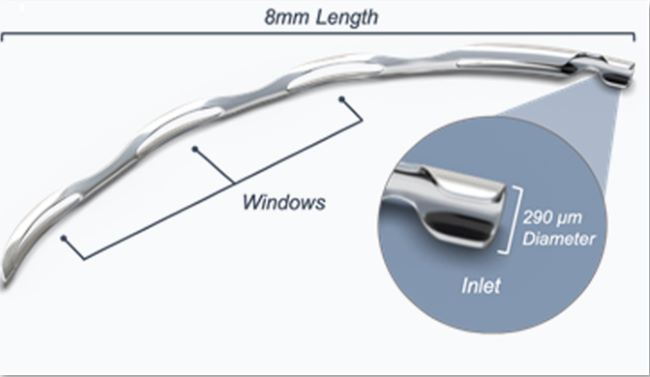
Figure 6. Hydrus stent.8
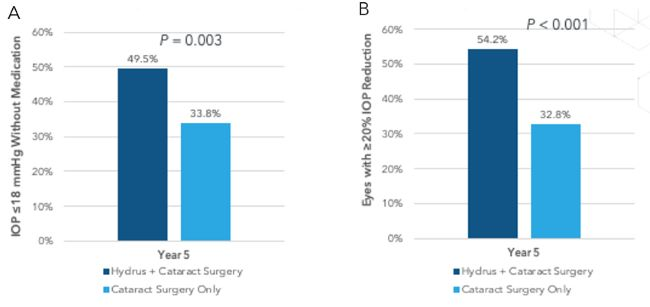
Figure 7. Hydrus efficacy of the proportion of eyes with (A) IOP <18 mmHg without medication and (B) eyes with >20% IOP reduction.9
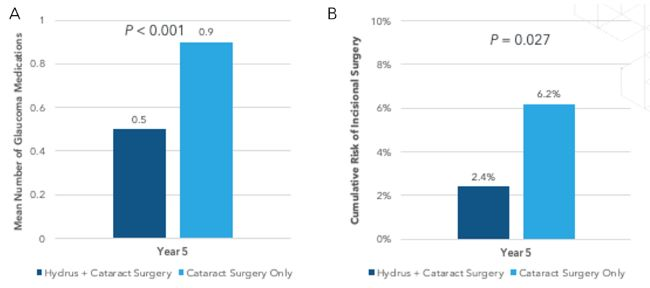
Figure 8. (A) Hydrus efficacy with mean number of glaucoma medications and (B) cumulative risk of incisional surgery after 5 years.9
iDoseTR
The iDoseTR is an intraocular implant in the late stages of development and is currently under FDA review. The implantable device slowly elutes travoprost to provide continuous drug delivery for patients with glaucoma (Figure 9). The implant is held into the angle in Schlemm’s canal through the trabecular meshwork. The device is designed to help improve compliance and adherence while minimizing the side effects of topical therapy.10
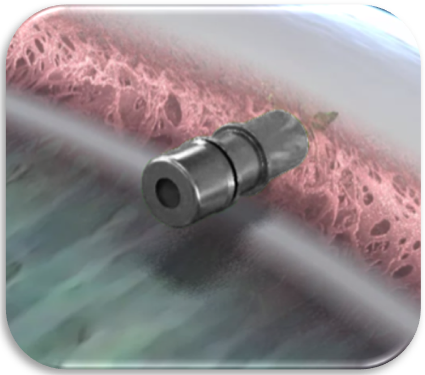
Figure 9. iDoseTR10
Two pivotal trials that are not yet published have shown noninferiority of iDose TR to timolol twice daily.11 The mean diurnal IOP reduction was between 6-8 mmHg, depending on the time of day. The studies included 1,150 subjects who were randomized in these trials. At baseline, the mean washed-out IOP was 24 mmHg, and 81% of study eyes had primary open-angle glaucoma, whereas the other 19% had ocular hypertension. A total of 67% of the randomized subjects were on at least 1 antiglaucoma medication, and 23% were on 2 or more. At 12 months, between 92% and 93% of eyes were controlled on the same or fewer medications compared to baseline. At 36 months, 69% were controlled. Interestingly, 81% of eyes were free of all glaucoma drops at 12 months.11
After 1 year for the phase 3 and 3 years for the phase 2B studies, there were no safety concerns, including no evidence of periorbitopathy, which is typically seen with prostaglandins; no significant endothelial cell loss; and no change in iris color.11,12 The iDoseTR can be implanted and exchanged, and a subset of patients had those procedures performed without any safety concerns or endothelial cell health concerns.12 The majority of the patients who underwent the exchange procedure did so in an operating room. However, a small group of patients had the exchange procedure performed in a minor procedure room in the office without any safety concerns.
Overall, the iDoseTR is a potential revolution in interventional glaucoma, and this is just the beginning of the sustained-release technology revolution. The results for safety and efficacy of the iDoseTR are very promising, and the future is bright for this type of technology. There will surely be more innovations in this space.
References
- Laroche D, Nkrumah G, Ng C. Real-world retrospective consecutive study of ab interno XEN 45 gel stent implant with mitomycin C in Black and Afro-Latino patients with glaucoma: 40% required secondary glaucoma surgery at 1 year. Middle East Afr J Ophthalmol. 2020;26(4):229-234.
- Reitsamer H, Vera V, Ruben S, et al. Three-year effectiveness and safety of the XEN gel stent as a solo procedure or in combination with phacoemulsification in open-angle glaucoma: a multicentre study. Acta Ophthalmol. 2022;100(1):e233-e245.
- Pinchuk L, Riss I, Batlle JF, et al. The use of poly(styrene-block-isobutylene-block-styrene) as a microshunt to treat glaucoma. Regen Biomater. 2016;3(2):137-142.
- Baker ND, Barnebey HS, Moster MR, et al. Ab-externo microshunt versus trabeculectomy in primary open-angle glaucoma: one-year results from a 2-year randomized, multicenter study. Ophthalmology. 2021;128(12):1710-1721.
- Sarkisian SR Jr, Grover DS, Gallardo MJ, et al. Effectiveness and safety of iStent Infinite trabecular micro-bypass for uncontrolled glaucoma. J Glaucoma. 2023;32(1):9-18.
- Samuelson TW, Sarkisian SR Jr, Lubeck DM, et al. Prospective, randomized, controlled pivotal trial of an ab interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract: two-year results. Ophthalmology. 2019;126(6):811-821.
- Ahmed IIK, Sheybani A, De Francesco T, et al. Long-term endothelial safety profile with iStent Inject in patients with open-angle glaucoma. Am J Ophthalmol. 2023;252:17-25.
- Samet S, Ong JA, Ahmed IIK. Hydrus microstent implantation for surgical management of glaucoma: a review of design, efficacy and safety. Eye Vis (Lond). 2019;6:32.
- Ahmed IIK, De Francesco T, Rhee D, et al. Long-term outcomes from the HORIZON randomized trial for a Schlemm's canal microstent in combination cataract and glaucoma surgery. Ophthalmology. 2022;129(7):742-751.
- Shouchane-Blum K, Geffen N, Zahavi A. Sustained drug delivery platforms – a new era for glaucoma treatment. Clin Exp Vis Eye Res. 2019;2(1):22-29.
- Hutton D. Glaukos announces results of two Phase 3 pivotal trials of iDose TR. Ophthalmology Times. September 9, 2022. https://www.ophthalmologytimes.com/view/glaukos-announces-results-of-two-phase-3-pivotal-trials-of-idose-tr
- Hutton D. Glaukos announces positive results for travoprost intraocular implant exchange trial, offers updates for corneal health pipeline programs. Ophthalmology Times. January 11, 2023. https://www.ophthalmologytimes.com/view/glaukos-announces-positive-results-for-travoprost-intraocular-implant-exchange-trial-offers-updates-for-corneal-health-pipeline-programs




Facebook Comments